Our Research Projects
We study the components of ubiquitin-proteasomal system (UPS) that are driving stem cell homeostasis and differentiation. Using hematopoietic stem cells as a model, we investigate how UPS influences stem cell fate decisions. Additionally, we develop targeted protein degradation tools to study protein function and eliminate cancer-associated proteins that are inaccessible to conventional drugs.
Hematopoietic stem cells
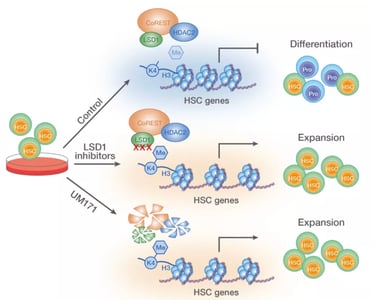
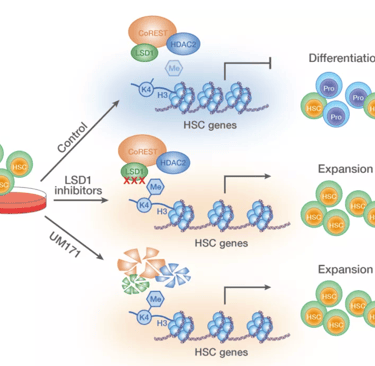
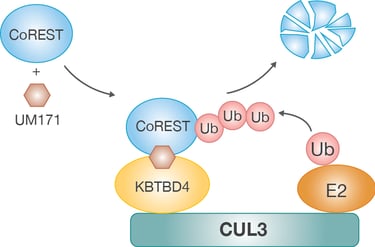
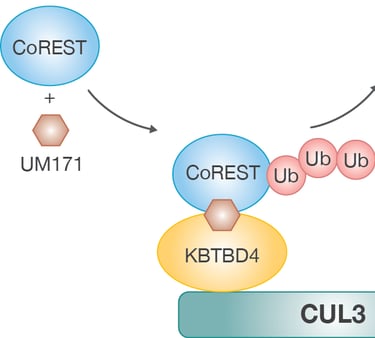
Our research focuses on how the ubiquitin-proteasomal system (UPS) regulates stem cell fate decisions by modulating the stability and activity of critical regulatory proteins. The UPS, consisting of a complex network of E3 ubiquitin ligases, precisely tags proteins for degradation or modification, thereby influencing vital processes such as cell cycle progression, signaling pathways, and the DNA damage response. By concentrating on hematopoietic stem cells, we seek to uncover how specific UPS components orchestrate the balance between self-renewal and differentiation. These findings deepen our understanding of stem cell biology and offer new possibilities for manipulating stem cell behavior in both regenerative medicine and disease treatment. Recently, we identified the molecular mechanism behind KBTBD4-UM171-mediated CoREST degradation, which acts as a key driver of human hematopoietic stem cell expansion at the expense of differentiation (Zemaitis et al., 2023, Journal of Biological Chemistry; Subramaniam et al., 2020, Blood).
Targeted protein degradation tools
We are developing protein degradation tools to target disease-associated proteins, with a particular focus on those involved in hematopoiesis and hematologic malignancies. By leveraging the precise substrate recognition capabilities of E3 ubiquitin ligases, we aim to design systems that selectively degrade proteins critical for cancer cell survival or resistance to therapy. We aim to use targeted degradation to eliminate proteins previously considered "undruggable." In the context of hematopoiesis, these strategies allow us to dissect the roles of specific proteins in stem cell self-renewal, differentiation, and lineage commitment, while also offering potential therapeutic solutions for blood-related diseases and cancers. Through this work, we strive to bridge fundamental research with translational applications, paving the way for novel treatments tailored to the unique challenges of hematologic disorders.
Funding















